At the recent annual BIO International Convention, more than 18,000 attendees converged on Boston to chart the biotech industry’s future and hold nearly 47,000 partnering meetings, a certified world record. The Innovation Institute was on hand to showcase its portfolio of life science discoveries available for licensing and to seek potential partnerships with industry, entrepreneurs and investors. Once again, the red hot field of cancer immunotherapy was front and center in a lot of the discussions. While in Boston, we took the opportunity to visit Pitt spinout, Oncorus, which is developing therapies in the emerging oncolytic virus domain of immunotherapy, to get an update on the company’s progress.
Photo by Richard Gayle Photography
Immunotherapies are re-shaping the way cancer is treated and leading to scores of new clinical trials to determine how cancer can be made detectable and treatable by the body’s immune system.
In the middle of a sea of biotech startups in Cambridge, MA, Oncorus Inc. is methodically working through its pre-clinical development of a technology platform licensed from Pitt from the lab of Joe Glorioso, professor of microbiology and molecular genetics. He is one of Pitt’s most prolific innovators and recipient of the 2017 Marlin Mickle Outstanding Innovator Award from the Innovation Institute.
Professor Glorioso is a pioneer in the genetic manipulation of herpes simplex virus (HSV) vectors engineered to infect tumor cells while avoiding healthy cells, resulting in tumor cell death which stimulates the body’s immune system to further attack the tumor.
Upon licensing the technology platform from Pitt, Oncorus raised $61 million in Series A financing in 2016.
The company continues pre-clinical development of these vectors with the goal of identifying a lead candidate drug in 2018 and filing an Investigative New Drug application in 2019. Over the past 18 months, Oncorus has hired a slew of biotech industry veterans who gravitated to the potential of the technology.
Christophe Quéva joined the company as Chief Scientific Officer (CSO) in October. Dr. Quéva most recently was CSO at iTeos Therapeutics, where he led the development of an immuno-oncology portfolio and previously held senior positions at AstraZeneca, Amgen and Gilead Sciences.
Dr. Quéva said that by 2019, Oncorus will seek to test its lead candidate in clinical trials as both a stand-alone treatment and in combination with checkpoint inhibitors that have been approved by the U.S. Food and Drug Administration (FDA). A checkpoint inhibitor blocks proteins made by cancer cells that reduce the body’s ability to mount an immune response against cancer. Oncorus’s platform is unique among other oncolytic viruses in development due to its large capacity to include additional agents that help to stimulate the immune response against the tumor.
Graphic courtesy of Oncorus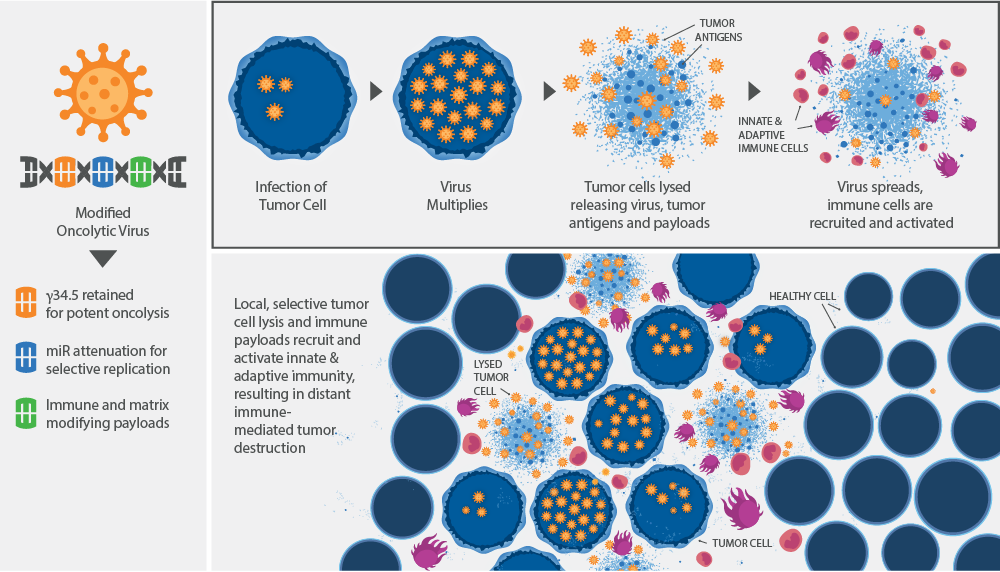
Oncorus's armed oncolytic viruses infect and conditionally replicate in tumor cells, resulting in tumor cell destruction
and the release of tumor antigens and therapeutic immune modulatory payloads into the tumor environment. Tumor antigens and immune payloads stimulate and recruit immune cells to create a potent personalized vaccine that destroys the primary tumor and distant metastasis, resulting in a highly effective cancer therapy.
In addition to oncolytic viruses and checkpoint inhibitors, other emerging immunotherapies include chimeric antigen receptor T-cell therapies, or CAR T-cell therapy, which involves harvesting a patient’s immune T cells and modifying them in the lab to attack cancer cells. These cells are then grown in the lab and infused back into the patient. Monoclonal antibodies are lab-made antibodies that can bind to cancer cells to treat cancer, both on their own and by delivering payload drugs or toxins directly to cancer cells. Vaccines are also being developed for several cancer types.
In general, oncolytic viruses are in an earlier stage of development, but they are attracting increasing interest from investors and other players in the biotech industry.
There is currently one oncolytic virus that has been approved by the FDA, Imlygic® (talimogene laherparepvec), marketed by Amgen, which is approved to treat advanced melanoma. It is now in a Phase III clinical trial in combination with Merck’s checkpoint inhibitor. In fact, there are now more than 1,000 trials of so-called combination therapies involving various immunotherapy treatments underway.
Recognizing the potential value that oncolytic virus technologies could add to their product portfolios, several pharma companies are acquiring or entering partnerships with oncolytic virus companies.
In May, Janssen Biotech, Inc., a division of Johnson and Johnson, acquired Rockland, MD-based privately-held oncolytic virus developer BeneVir Biopharm, Inc., in a deal worth up to $1 billion in milestones.
In February, Merck paid nearly $400 million to acquire an Australian oncolytic virus company, Viralytics, which is in clinical trial as a combination therapy with Merck’s Keytruda in several cancer types.
Bristol-Myers Squibb is in a development agreement with UK-based oncolytic virus developer PsiOxus Therapeutics, which has recently received approval to move to human trials.
Pitt has another spinout in the oncolytic virus space, Western Oncolytics, which inked a development partnership with pharma giant Pfizer in 2015. Pfizer is supporting Western Oncolytics’s pre-clinical development and will be examining its lead target in combination with other immunotherapies in its portfolio. Western Oncolytics recently became one of the inaugural companies to join the LifeX life sciences accelerator in Pittsburgh’s Strip District.
Oncorus’s first virus to enter the clinic is designed for delivery within the tumor to expose the patient’s own tumor antigens and to stimulate the patient’s own immune response against the tumor. “The beauty of oncolytic viruses is to provide a personalized medicine with an ‘off-the-shelf’ product,” said Dr. Quéva. Oncorus is also exploring other innovative approaches such as those that will enable intravenous administration to reach distant metastatic sites that may be difficult to inject, where he said the unmet needs are significant, particularly in cancers that poorly respond to immune checkpoint inhibitors.
If you are a Pitt faculty or student innovator who has a discovery with commercial potential, there are many resources available through the Innovation Institute to begin your journey from the lab to the market. Innovation Igniter is a two-hour workshop that introduces you to the commercialization process. The next Igniter is Wednesday, August 15, 10 a.m. - 12 p.m. at the CMU Project Olympus incubator, 4620 Henry Street, Pittsburgh, PA 15213.
You can contact one of our Entrepreneurs in Residence at any time to discuss your innovation and how to move it forward. Click here to view their areas of expertise.